Research Interests
The Innate Immune Response to Viral Infections
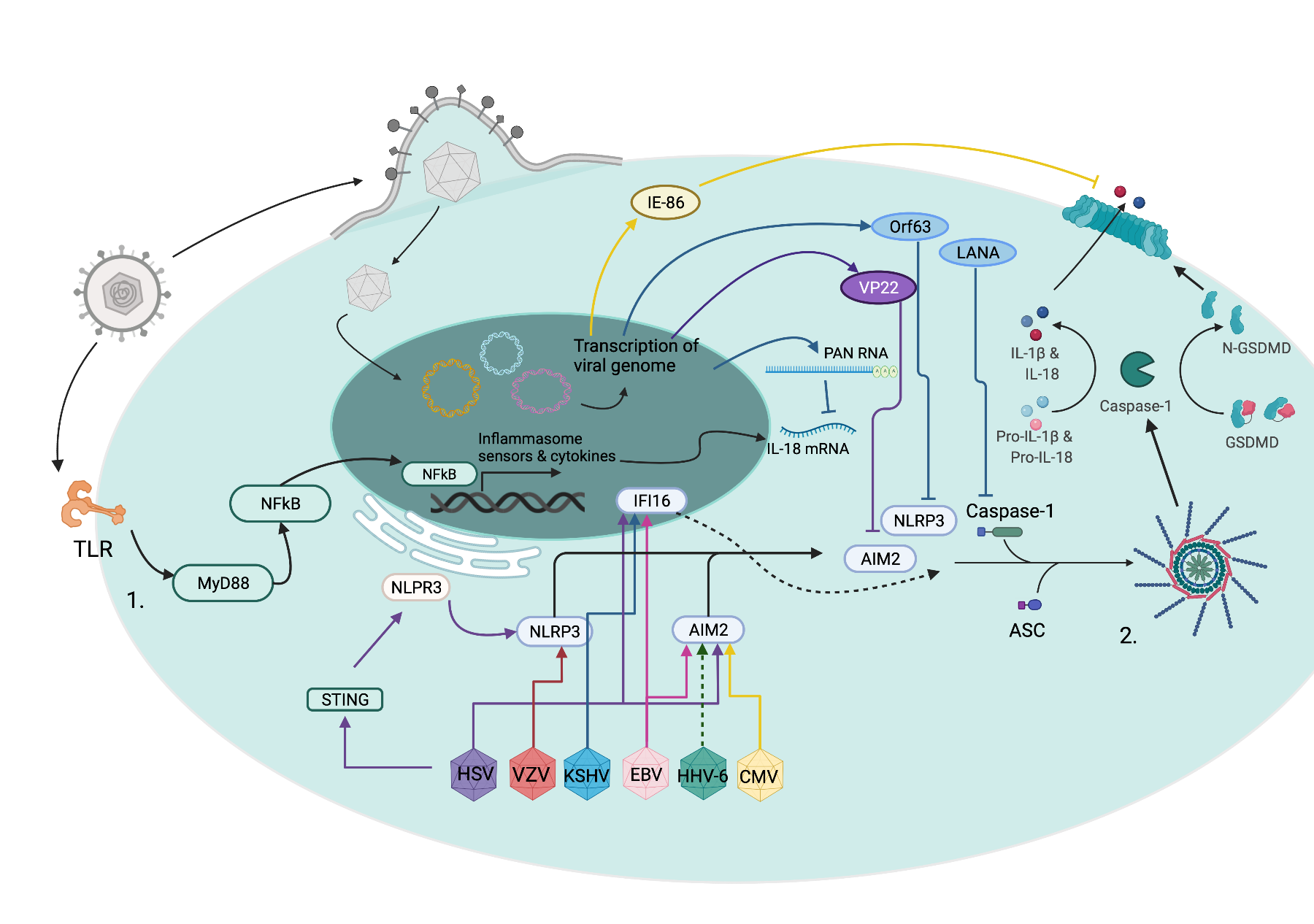
The innate immune response is the body’s first defense against pathogens. My lab is interested in understanding how viruses activate and subvert this defensive system. We work in a large collaborative environment studying herpes simplex virus, cytomegalovirus, SARS-CoV-2, RSV, and influenza. Current areas of investigation include how these viruses activate inflammatory pathways, alter innate immune cell signaling, and interact with novel regulators of inflammation.
Vaccine Response in Immunosuppressed Hosts
Vaccines remain our best tool to prevent infection and disease. Unfortunately, people with immunocompromising conditions (bone marrow or organ transplant recipients) often do not respond as well as immunocompetent individuals to vaccines. This leaves them vulnerable to infection. My lab works with other investigators to understand the vaccine response in these immunosuppressed patients and how we can improve that response to protect them from disease.
Open Research Positions
Transplant/Oncology Infectious Diseases
My primary clinical focus is on preventing and treating infections in immunosuppressed patients. This includes bone marrow transplant recipients, solid organ transplant recipients, and patients undergoing chemotherapy treatment for cancer. I see patients at Johns Hopkins Hospital and affiliated clinics. I have a particular interest in novel treatments for viral infections in this population.
Selected Publications
- Karaba AH, Hage C, Sengsouk I, Balasubramanian P, Segev DL, Tobian AAAR, Werbel WA. Antibody Response to Respiratory Syncytial Virus Vaccination in Immunocompromised Persons. JAMA. 2024. doi:10.1001/jama.2024.25395.
- Hsieh LL, Looney M, Figueroa A, Massaccesi G, Stavrakis G, Anaya EU, D’Alessio FR, Ordonez AA, Pekosz AS, DeFilippis VR, Karakousis PC, Karaba AH, Cox AL. Bystander monocytic cells drive infection-independent NLRP3 inflammasome response to SARS-CoV-2. mBio. 15:e00810-24. doi.org/10.1128/mbio.00810-24.
- Johnston TS, Hage C, Abedon AT, Panda S, Alejo JL, Eby Y, Segev DL, Tobian AAT, Cox AL, Werbel WA, Karaba AH. Rapid Wane and Recovery of XBB Sublineage Neutralization After Sequential Omicron-based Vaccination in Solid Organ Transplant Recipients. Clinical Infectious Diseases. 2024:ciae279. doi:10.1093/cid/ciae279
- Karaba AH, Morgenlander WR, Johnston TS, Hage C, Pekosz A, Durand CM, Segev DL, et al. Epitope Mapping of SARS-CoV-2 Spike Antibodies in Vaccinated Kidney Transplant Recipients Reveals Poor Spike Coverage Compared to Healthy Controls. The Journal of Infectious Diseases. 2023, jiad534. https://doi.org/10.1093/infdis/jiad534.
- Karaba AH, Johnston TS, Beck E, Laeyendecker O, Cox AL, Klein SL, Sullivan DJ. Endemic Human Coronavirus Antibody Levels Are Unchanged after Convalescent or Control Plasma Transfusion for Early Outpatient COVID-19 Treatment. mBio. 2023:e03287-22. doi:10.1128/mbio.03287-22
- Karaba AH, Zhou W, Li S, Aytenfisu TY, Johnston TS, Akinde O, et al. Impact of Seasonal Coronavirus Antibodies on SARS-CoV-2 Vaccine Responses in Solid Organ Transplant Recipients. Clinical Infectious Diseases. 2022; ciac652. doi:10.1093/cid/ciac652
- Kumar A, Stavrakis G, Karaba AH. Herpesviruses and Inflammasomes: One Sensor Does Not Fit All. Szpara ML, Prasad VR, editors. mBio. 2022; e01737-21. doi:10.1128/mbio.01737-21
- Karaba AH, Zhu X, Benner SE, Akinde O, Eby Y, Wang KH, et al. Higher Proinflammatory Cytokines Are Associated With Increased Antibody Titer After a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients. Transplantation. 2022;106: 835–841. doi:10.1097/TP.0000000000004057
- Karaba AH, Figueroa A, Werbel WA, Dioverti MV, Steinke SM, Ray SC, et al. Interleukin-18 and tumor necrosis factor-α are elevated in solid organ transplant recipients with possible cytomegalovirus end-organ disease. Transplant Infectious Disease. 2021. doi:10.1111/tid.13682
- Karaba AH, Zhou W, Hsieh LL, Figueroa A, Massaccesi G, Rothman RE, et al. Differential Cytokine Signatures of SARS-CoV-2 and Influenza Infection Highlight Key Differences in Pathobiology. Clinical Infectious Diseases. 2021. doi:10.1093/cid/ciab376
- Karaba AH, Figueroa A, Massaccesi G, Botto S, DeFilippis VR, Cox AL. Herpes simplex virus type 1 inflammasome activation in proinflammatory human macrophages is dependent on NLRP3, ASC, and caspase-1. PLOS ONE. 2020;15: e0229570. doi:10.1371/journal.pone.0229570
© 2025 all rights reserved.
This website was made using the Distill package in R.
Learn more about using Distill for R Markdown at https://rstudio.github.io/distill